When you hear the word tazarotene, you probably think of acne patches or prescription creams from your dermatologist. But the drug is now at the center of a wave of innovation that could reshape how we treat everything from wrinkles to hyperpigmentation. In this article we’ll break down what tazarotene is, why it works so well today, and the tech‑driven tweaks that could make it even more powerful in the next few years.
Key Takeaways
- Tazarotene is a third‑generation retinoid that binds to retinoic‑acid receptors with high selectivity.
- It’s approved for acne, plaque psoriasis, and facial photodamage, and is increasingly used off‑label for melasma.
- New delivery systems-nanoparticles, liposomes, microneedle patches-aim to boost skin penetration while cutting irritation.
- Combination regimens (e.g., with benzoyl peroxide or hyaluronic acid) are showing faster results in early trials.
- Regulators are reviewing next‑gen retinoid analogs that could be marketed as over‑the‑counter options by 2027.
Understanding Tazarotene
Tazarotene is a synthetic, third‑generation retinoid that selectively activates retinoic‑acid receptors RAR‑γ and RAR‑β. By binding these receptors, it normalizes keratinocyte differentiation, reduces follicular hyperkeratinization, and modulates inflammatory pathways. Compared with older retinoids like tretinoin, tazarotene’s molecular structure (a thioester side chain) gives it greater stability in topical formulations and a stronger affinity for the skin‑specific receptors that drive therapeutic effects.
Current Clinical Uses
Since its FDA approval in 2000, tazarotene has become a workhorse in dermatology. The three biggest indications are:
- Acne vulgaris - 0.1% and 0.05% gels reduce inflammatory lesions by up to 70% after 12 weeks.
- Plaque psoriasis - once‑daily cream improves PASI scores by an average of 4 points in moderate disease.
- Photodamage and fine lines - the 0.1% cream stimulates collagen synthesis, leading to measurable skin‑thickness gains in clinical trials.
Off‑label, many clinicians combine tazarotene with hydroquinone for melasma, leveraging its ability to speed up epidermal turnover and dilute pigment deposits.
Safety & Side Effects
Because tazarotene works at the cellular level, irritation is its most common adverse event. Patients typically report erythema, peeling, and a transient burning sensation during the first two weeks. To mitigate these effects, dermatologists advise a “start low, go slow” approach-applying a pea‑sized amount every other night and increasing frequency as tolerance builds.
Systemic absorption is minimal; serum levels remain below 0.1 ng/mL, well under the threshold that would trigger retinoid syndrome. However, pregnant women should avoid any topical retinoid due to teratogenic risk.
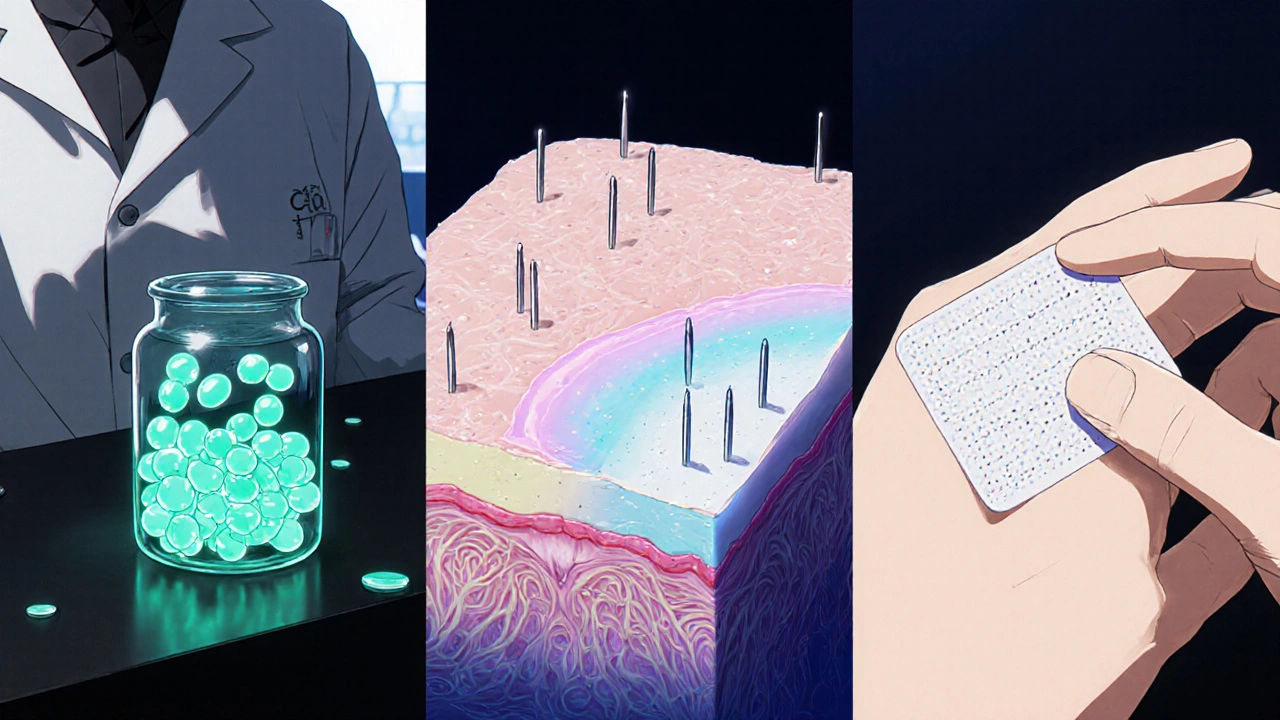
Emerging Formulations
Today's biggest research frontier is how to get more drug into the deeper dermis while keeping the surface calm. Three delivery platforms dominate the pipeline:
- Nanoparticle Delivery - lipid‑based nanocarriers encapsulate tazarotene, boosting penetration by up to 3‑fold in ex‑vivo skin models. A 2023 Phase II trial showed 30% less erythema compared with conventional gel.
- Liposomal Formulation - phospholipid vesicles protect the active from oxidation and release it slowly, extending the drug’s half‑life on the skin from 4 hours to roughly 12 hours.
- Microneedle Patch - a biodegradable array creates micro‑channels that bypass the stratum corneum. Early human data suggest a 50% reduction in treatment time (once‑weekly patches versus daily cream) without a rise in adverse events.
All three technologies are expected to enter the market between 2026 and 2028, offering dermatologist‑prescribed options that balance potency with comfort.
Combination Strategies
Combining tazarotene with other actives can accelerate results and address multiple skin concerns at once. The most studied pairings are:
- Benzoyl Peroxide - kills P. acnes bacteria while tazarotene clears clogged pores. A 2022 split‑face study reported a 2‑point drop in IGA score within 4 weeks compared to tazarotene alone.
- Hyaluronic Acid Serum - replenishes moisture, reducing transepidermal water loss and making peeling less noticeable.
- Vitamin C - antioxidant protection helps prevent the post‑inflammatory hyperpigmentation that sometimes follows aggressive retinoid use.
When layering, the rule of thumb is to apply tazarotene first (clean, dry skin), wait 15 minutes, then add the secondary product. This sequence ensures optimal receptor binding.

Future Landscape
Looking ahead, three big trends could redefine tazarotene’s place in skincare:
| Attribute | Tazarotene | Adapalene | Tretinoin |
|---|---|---|---|
| Receptor Selectivity | High (RAR‑γ/β) | Moderate | Broad |
| Typical Irritation Rate | 30‑40% | 20‑30% | 45‑55% |
| Stability in Creams | Excellent | Good | Poor |
| Approved Indications | Acne, Psoriasis, Photodamage | Acne | Acne, Anti‑aging (off‑label) |
| OTC Availability (US) | Prescription | Prescription | Prescription |
Beyond formulation tweaks, scientists are engineering next‑generation retinoid analogs that retain the skin‑specific receptor binding of tazarotene but have an even lower irritation profile. Early animal studies show up to a 60% reduction in erythema with comparable efficacy.
Artificial‑intelligence driven design platforms are also predicting optimal side‑chain modifications, cutting the discovery timeline from years to months. By 2027, we might see an OTC‑ready “soft‑tazarotene” that consumers can buy without a prescription, much like existing retinol products.
Regulatory bodies such as the FDA have opened a fast‑track pathway for topical retinoids that demonstrate a risk‑benefit ratio favoring over‑the‑counter status. The first applications are slated for review in early 2026.
Practical Guide for Consumers
If you’re considering tazarotene, follow this simple roadmap:
- Consult a dermatologist - get a prescription and confirm that your skin type can tolerate a retinoid.
- Start slow - apply a pea‑sized amount every other night for two weeks.
- Moisturize - use a fragrance‑free moisturizer 15 minutes after the drug to seal in hydration.
- Protect - wear SPF 30+ daily; retinoids increase photosensitivity.
- Monitor - if severe peeling occurs, reduce frequency or switch to a lower concentration (0.05%).
Remember, visible improvements usually appear after 8-12 weeks. Patience is key; the long‑term collagen‑boosting benefits far outweigh the short‑term redness.
Frequently Asked Questions
Can I use tazarotene if I have sensitive skin?
Yes, but begin with the 0.05% formula and apply every third night. Pair it with a gentle moisturizer to reduce irritation.
Is tazarotene safe during pregnancy?
No. Topical retinoids are classified as Category X for pregnancy. Discuss alternative treatments with your doctor.
How does tazarotene differ from over‑the‑counter retinol?
Tazarotene is a prescription‑strength retinoid that directly activates retinoic‑acid receptors, delivering faster and more pronounced results. Retinol must first convert to retinaldehyde and then to retinoic acid, making it less potent.
Will the new nanoparticle creams be available in Australia?
Several Australian dermatology clinics are enrolling patients in Phase III trials slated for 2026, so commercial release is expected by late 2027.
Can I combine tazarotene with other prescription acne meds?
Doctors often pair it with oral isotretinoin or oral antibiotics for severe cases, but close monitoring is essential to avoid excessive dryness.









Suraj 1120
24 Oct 2025 at 12:20The hype around tazarotene’s new nano‑carriers is overblown. Clinical data still shows a 30‑40% irritation rate, and the marginal boost in penetration doesn’t justify the extra cost. Most dermatologists will stick to the tried‑and‑true gel until the safety profile improves. Bottom line: it’s a marketing gimmick for now.