When a brand-name drug’s patent expires, the promise of cheaper generics kicks in. Patients expect prices to drop. Pharmacies expect more options. But what if the brand company itself launches a generic version-same pill, same factory, same price-and steals the first generic’s thunder? That’s what authorized generics do. And they’re changing how competition works in the drug market-not for the better.
What Exactly Is an Authorized Generic?
An authorized generic isn’t some new drug. It’s the exact same medicine as the brand-name version, just repackaged under a generic label. No new testing. No new approval. The same factory, same ingredients, same bottle-except now it says "simvastatin" instead of "Zocor." The brand company makes it, sells it, and sometimes even lets another company distribute it under license. The FDA allows it because the law doesn’t say they can’t. Here’s the catch: this happens right when the first independent generic company is supposed to get its 180-day monopoly on selling the generic version. That exclusivity period is the whole reason generic companies spend millions suing brand companies over patents. They bet they’ll get a clear shot at the market. But if the brand drops its own generic right after, that bet collapses.How Authorized Generics Kill the Incentive to Challenge Patents
The Hatch-Waxman Act of 1984 was meant to speed up generic access. It gave the first generic company to challenge a patent 180 days of exclusive sales. That’s a huge reward. It’s how companies like Teva and Mylan made billions. But authorized generics turn that reward into a trap. The FTC found that when an authorized generic enters the market, the first generic’s revenue drops by 40 to 52% during those 180 days. Without an authorized generic, that first filer would grab 80 to 90% of the generic market. With one? They’re lucky to get 30%. And it doesn’t stop there. In the next 30 months, their revenue stays 53 to 62% lower than if no authorized generic had ever shown up. Think about it: a generic company spends $10 million on legal fees to win a patent lawsuit. They expect to make $100 million in the next six months. Then the brand company drops its own generic at 15% below the brand price-but still 25% above the true generic. Suddenly, the generic company’s profit vanishes. Why risk $10 million for $5 million in returns?The Quiet Deal: Reverse Payments and Authorized Generics
It gets worse. Sometimes, the brand company doesn’t even bother launching its own generic. Instead, they pay the first generic company to stay out of the market. And in return? The brand promises not to launch an authorized generic. It’s called a "reverse payment settlement." Between 2004 and 2010, about 25% of patent settlements involving first-filer generics included this kind of deal. These weren’t small payments. They involved drugs worth over $23 billion in annual sales. The result? Generic entry got delayed by an average of 37.9 months. That’s more than three years of monopoly pricing for the brand. The FTC called this the "most egregious form of anti-competitive behavior." The Supreme Court agreed in 2013, ruling that reverse payments need antitrust scrutiny. But authorized generics slipped through the cracks. Courts didn’t ban them. Regulators didn’t stop them. They just kept watching.
Who Benefits? Who Loses?
Branded drug companies win. They keep control. They get to set the price floor. They avoid losing market share to a true competitor. They even get to collect revenue from their own generic-sometimes through a shell subsidiary-while pretending they’re "supporting competition." Pharmacy benefit managers (PBMs) often say they like authorized generics because they add another pricing tier. A 2023 survey found 68% of PBM executives prefer formularies that include them. But that’s not because they’re cheaper. It’s because they’re predictable. PBMs can negotiate with one company-the brand-instead of two. Patients? They don’t see the full picture. Sure, an authorized generic might be cheaper than the brand. But it’s still way more expensive than the real generic that never got to launch. And when fewer companies challenge patents because the payoff is gone, fewer true generics ever come to market. That means higher prices long-term. Independent generic manufacturers are getting crushed. Teva reported a $275 million revenue loss in 2018 just from one product hit by an authorized generic. The Generic Pharmaceutical Association (now the Association for Accessible Medicines) has been fighting this for years. Their argument is simple: if you destroy the 180-day exclusivity, you destroy the incentive to challenge patents. And without those challenges, patents last longer. Prices stay high.Why This Isn’t Really Competition
PhRMA, the big pharma lobby, says authorized generics increase competition. They cite a 2024 Health Affairs study claiming pharmacy prices dropped 13-18% when an authorized generic was available. But that’s misleading. The drop isn’t because of true competition. It’s because the brand company lowered its own price slightly to compete with itself. Real competition means two separate companies, with different costs, different incentives, different strategies, fighting over price. An authorized generic isn’t a separate company. It’s the same entity with a different label. It’s like a carmaker launching a "budget version" of its own luxury sedan-same factory, same engineers, same profit margin. It doesn’t create competition. It creates a controlled market. The FTC has been clear: authorized generics distort the system Congress designed. The Hatch-Waxman Act created a balance: patents protect innovation, but generics break the monopoly. Authorized generics break that balance. They let the patent holder keep control while pretending they’re helping.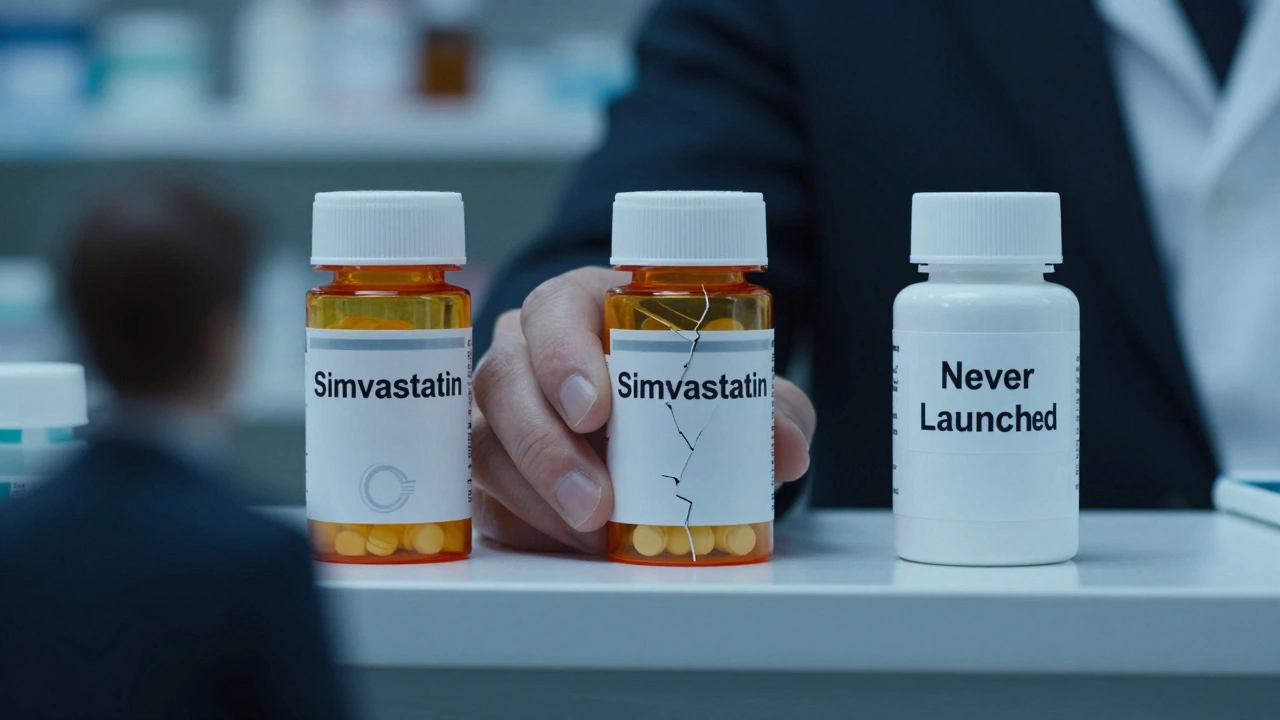
The Trend Is Changing-But Not Because of Fairness
Here’s something new: authorized generics are becoming less common. In 2010, they showed up in 42% of markets with first-filer exclusivity. By 2022, that number dropped to 28%. Why? Not because companies got ethical. It’s because regulators are watching. The FTC has opened 17 investigations since 2020. In 2022, they made it clear: "We will challenge any arrangement that uses authorized generics to circumvent the competitive structure Congress established." Congress is trying too. Senators Klobuchar and Grassley reintroduced the Preserve Access to Affordable Generics and Biosimilars Act in 2023. It would ban deals that delay authorized generic entry. Companies are adapting. Instead of outright deals, they’re using more complex timing. Some wait until after the 180-day window. Others delay launch until after a court ruling. But the goal stays the same: control the market without breaking the law.What This Means for You
If you’re a patient, you might see lower prices on some generics. But don’t assume it’s because of competition. It’s because the brand company is playing a game. The real savings-the ones that come from multiple independent manufacturers competing-never happen. If you’re a pharmacist or a health plan, you might be tempted to favor authorized generics because they’re reliable. But ask yourself: are you helping patients, or just making it easier for the brand to keep control? If you’re someone who believes in fair competition, this is a warning. The system was built to give power to the underdog-the small generic company challenging a giant. Authorized generics turn that underdog into a pawn. And when the underdogs stop showing up, the giants win. Always.What’s Next?
The next few years will decide if authorized generics become a permanent feature-or a fading tactic. The FTC is pushing harder. Courts are watching. Congress might finally act. But until then, the same pattern repeats: a patent expires, a generic sues, the brand counters with a generic of its own, and the real competition disappears. The question isn’t whether authorized generics are legal. They are. The question is: should they be allowed to break the system Congress designed to save patients money?Are authorized generics the same as regular generics?
Yes and no. Authorized generics contain the exact same active ingredients, dosage, and manufacturing process as the brand-name drug. The only difference is the label and packaging-they’re sold under a generic name. Regular generics, on the other hand, are made by separate companies that reverse-engineered the drug and got FDA approval independently. They’re not made by the brand company.
Why do brand companies launch authorized generics?
To protect their profits. When a generic company wins the right to be first to market, it can charge much lower prices and capture most of the sales. By launching its own generic, the brand company splits that market. It keeps control over pricing and prevents the independent generic from dominating. It’s not about lowering prices-it’s about controlling them.
Do authorized generics lower drug prices for consumers?
Sometimes, but not in the way you think. Authorized generics are usually cheaper than the brand name, but still significantly more expensive than true generics. The real price drops come when multiple independent generics enter the market. Authorized generics block that by reducing the incentive for other companies to enter. So while you might see a small discount, the bigger, lasting savings never happen.
Is it legal for a brand company to launch an authorized generic during the 180-day exclusivity period?
Yes. The FDA and courts have consistently ruled that the Hatch-Waxman Act doesn’t prohibit it. The law gives the first generic company exclusivity, but it doesn’t stop the brand company from selling its own version under a generic label. That legal loophole is why this practice continues, even though it undermines the law’s intent.
What’s being done to stop authorized generics from hurting competition?
The FTC is actively investigating agreements where brand companies promise not to launch an authorized generic in exchange for the generic company delaying entry. Congress has introduced bills like the Preserve Access to Affordable Generics and Biosimilars Act to ban such deals. While no law has passed yet, regulatory pressure is increasing, and companies are becoming more cautious about using authorized generics in settlements.







Jamie Clark
13 Dec 2025 at 23:19They call this 'competition'? It's corporate theater. The brand company doesn't want to lose market share-they want to own the entire game, even when the rules say they shouldn't. This isn't capitalism. It's feudalism with pill bottles.